How Many Total Electrons Does Oxygen Have
Electron configuration is the arrangement of electrons on the orbitals. 13 How many valence electrons does xenon have.

How To Find Valency What Are Valence Electrons Teachoo
Oxygen is 8 in the periodical table.
. The number of neutrons is equal to the Atomic Mass minus the Atomic Number. 17 How many shells are in helium. So there are 16 - 8 8 neutrons.
15 How many electrons are there in carbon. So oxygen has a strong tendency to gain two electrons from the other electron donating species and thus it acts as a strong Lewis acid. That is an oxygen atom has a total of eight electrons.
18O2 has 8 protons 10 neutrons and 10 electrons. Therefore the O electron configuration will be 1s22s22p4. 8 Why does C have 4 valence electrons.
Oxygen has an electron arrangement of 2 6 and needs to gain two electrons to fill the n2 energy level and achieve an octet of electrons in the outermost shell. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f. Carbon has 4 valence electrons.
The Oxygen atom is made of 8 proton 8 electrons and generally 8 neutrons. There are 8 electrons in the oxygen atom but as an oxygen ion is O 2- then there are 10 electrons in an oxygen ion. 14 How many electrons does carbon have in total.
That is we can finally say that there are electrons equal to the atomic number in the oxygen atom. 10 How many sublevels are there with n 3 in an atom. Oxygen being a chalcogen has six electrons in its outermost shell and requires only two electrons to complete its octet.
16 How do you find orbital shells. 14 What is the orbital notation of GE. Therefore oxygen has 8 electrons.
Oxygen has 6 valence electrons. From the periodic table we see that the atomic number of oxygen is 8. Thus the total number of valence electrons present in an oxygen atom is six.
6 How many electrons can have n 5 and L 3. 9 What is the maximum number of electrons possible with N 3 in an atom. 12 How many neutrons does GE have.
How many valence electrons are in the oxygen ion. 12 How many shells does hydrogen have. 10 How many energy levels and valence electrons does an atom of germanium Ge have.
The oxygen atom has a total of 8 electrons so we have to put 8 electrons in orbitals. The way to determine this is to look at the atomic number of oxygen which is 8. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital.
10 How many electrons does carbon have in its outer shell. Together it would be 10 valence electrons. The oxygen ion is formed when the oxygen atom gains two valence electrons.
First electron shell can hold 2 electrons. Valence electrons are the ones on the outermost shell there are some exceptions but they are much further down the table. A neutral atom of oxygen-18 will have 8 electrons.
The number of neutrons normally varies and can be calculated from the atomic weight. The electrons will be placed in different orbitals according to the energy level. This results in an oxygen ion with a 2- charge.
And electrons equal to protons are located outside the nucleus. An oxygen atom has two electron shells and 6 valence electrons. 7 How many electrons in an atom could have these sets of quantum numbers n5.
1 4 2 6 3 8 4 16. 9 How many 4s electrons are in GE. How Many Total Electrons Are In A Cu2 Ion27 electronsHow many ions are in Cu2How many total electrons does a copper I ion haveA copper ion with a charge of 2 has 29 protons and 27 electrons.
It would leave 4 pairs left. How many electrons does oxygen-18 have. 13 Why is 3rd shell 8 or 18.
Therefore oxygen has 8 electrons. 8 What is the maximum number of electrons that can have n 3. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital.
Which of the following subatomic particles do NOT have a charge. How many total electrons does this atom have. 1 electrons 2 neutrons 3 ions 4 protons.
The atomic number of an element is equal to the number of protons in the nucleus of each atom of that. 11 How many 4p electrons are in GE. So for your question the Periodic Table tells us that oxygen has an Atomic Number of 8 so there are 8 protons and 8 electrons.
An oxygen atom is what is found on the periodic table. 15 How many valence does El have. The Periodic Table tells us that oxygen has an Atomic Mass of 16.
Oxygen is the eighth element with a total of 8 electrons. The number of protons and the number of electrons are always the same in an element that is neutral and has no charge. The oxide ion will have a charge of 2 as a result of gaining two electrons.
11 Does carbon have a full shell. Therefore out of 8 electrons 2 go to the first shell and 6 to the second.
What Is The Number Of Valence Electrons In Oxygen Socratic

How Many Valence Electrons Does Oxygen O Have

Interpreting Atomic Structure Ppt Video Online Download
How Many Protons Neutrons And Electrons Does Oxygen Have Quora
An Atom Of Oxygen Has Six Valence Electrons In Nature Oxygen Is A Diatomic Molecule And Is Usually Found In The Form O2 Why Would One Atom Of Oxygen Want To Bond
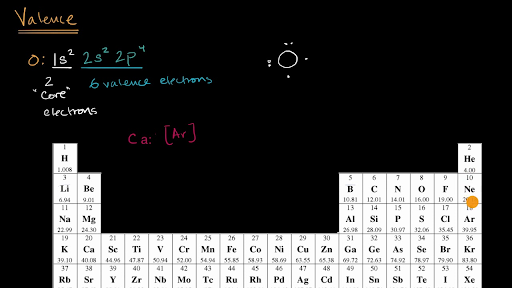
Valence Electrons Video Khan Academy

Oxygen Bohr Model How To Draw Bohr Diagram For Oxygen O Atom
How To Figure Out How Many Electrons Oxygen Has If It Has 6 Protons And 6 Neutrons Quora

How Many Electrons Does Oxygen Have In Its Outermost Shell
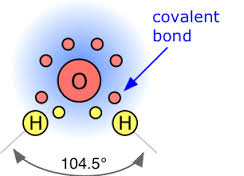
The Chemistry Of Water Aqua Health Products

How Many Valence Electrons Does Oxygen O Have
How Many Electrons Are There In An Oxygen Molecule Quora
8 Atoms Tend To Form Bonds Until Their Valence Electron Shell Is Filled Labxchange

How Many Valence Electrons Does Oxygen O Have

How Many Protons And How Many Electrons Are In An Oxygen Ion That Has A 2 Charge

How Many Valence Electrons Does Oxygen O Have

Oxygen Bohr Model How To Draw Bohr Diagram For Oxygen O Atom

Let S Review Oxygen O 8 1s2 2s2 2p4 Oxygen Has 6 Valence Electrons Ppt Download
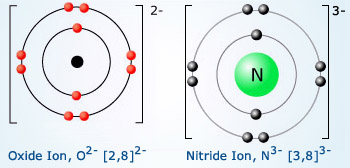
Oxygen Valence Electrons Oxygen Valency Electron Configuration
Comments
Post a Comment